
The viscosity of an aqueous solution can either increase or decrease with concentration depending on the solute and the range of concentration. This is also the reason oils tend to be highly viscous, since they are usually composed of long-chain hydrocarbons. More dramatically, a long-chain hydrocarbon like squalene (C 30H 62) has a viscosity an order of magnitude larger than the shorter n-alkanes (roughly 31 mPa This effect can be observed for the n-alkanes and 1-chloroalkanes tabulated below. Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow. One of the key predictions of the theory is the following relationship between viscosity μ For this reason, measured viscosities of the noble gases serve as important tests of the kinetic-molecular theory of transport processes in gases (see Chapman–Enskog theory). The simple structure of noble gas molecules makes them amenable to accurate theoretical treatment. By contrast, pressure is omitted since gaseous viscosity depends only weakly on it. The temperatures corresponding to each data point are stated explicitly. Where data points are unavailable for 25 ☌ or 1 atmosphere, values are given at a nearby temperature/pressure. Here "standard conditions" refers to temperatures of 25 ☌ and pressures of 1 atmosphere. Viscosities at or near standard conditions Consequently, its kinematic viscosity is around 2 to 40 centiStokes. The density is usually on the order of 0.5 to 5 kg/m^3. Consequently, if a liquid has dynamic viscosity of n centiPoise, and its density is not too different from that of water, then its kinematic viscosity is around n centiStokes.įor gas, the dynamic viscosity is usually in the range of 10 to 20 microPascal-seconds, or 0.01 to 0.02 centiPoise. The density is usually on the order of 1000 kg/m^3, i.e. In engineering, the unit is usually Stoke or centiStoke, with 1 Stoke = 0.0001 m^2/s, and 1 centiStoke = 0.01 Stoke.įor liquid, the dynamic viscosity is usually in the range of 0.001 to 1 Pascal-second, or 1 to 1000 centiPoise. In engineering, the unit is usually Poise or centiPoise, with 1 Poise = 0.1 Pascal-second, and 1 centiPoise = 0.01 Poise.įor kinematic viscosity, the SI unit is m^2/s. This page lists only dynamic viscosity.įor dynamic viscosity, the SI unit is Pascal-second. Kinematic viscosity is dynamic viscosity divided by fluid density. The values listed in this article are representative estimates only, as they do not account for measurement uncertainties, variability in material definitions, or non-Newtonian behavior. Of all fluids, gases have the lowest viscosities, and thick liquids have the highest.
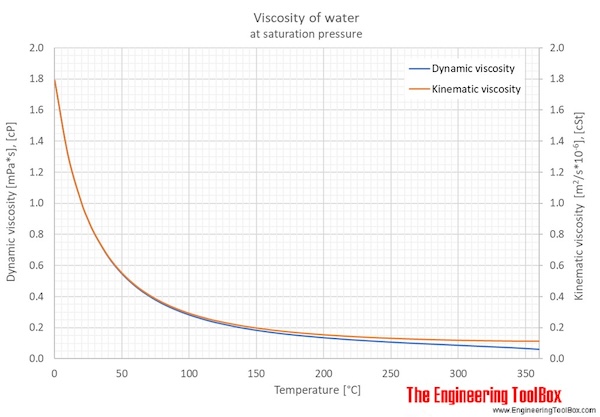
Viscosity is measured using a viscometer. For instance, honey hasĪ much higher viscosity than water. It corresponds roughly to the intuitive notion of a fluid's 'thickness'. Dynamic viscosity is a material property which describes the resistance of a fluid to shearing flows.


 0 kommentar(er)
0 kommentar(er)
